Pobierz materiał i Publikuj za darmo

OTTAWA and BOSTON and CARLSBAD, Calif., Oct. 04, 2018 (GLOBE NEWSWIRE) - Akcea Therapeutics Inc. (NASDAQ: AKCA), an affiliate of Ionis Pharmaceuticals, Inc., and Ionis Pharmaceuticals, Inc. (NASDAQ: IONS), announced today that TEGSEDITM (inotersen injection) is now approved in Canada for the treatment of stage 1 or stage 2 polyneuropathy in adult patients with hereditary transthyretin amyloidosis (hATTR) (1).
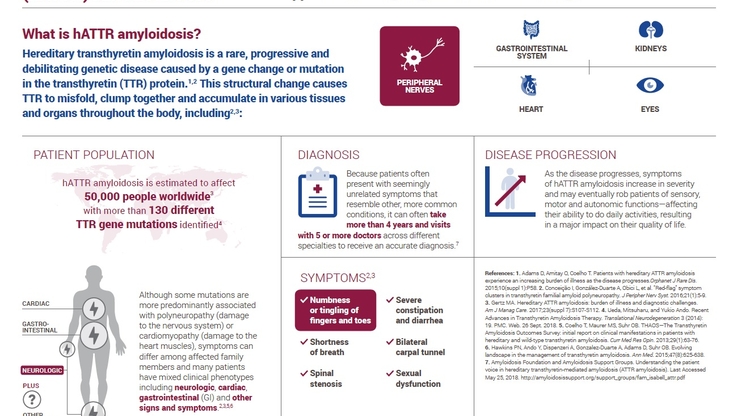
Following a priority review by Health Canada, TEGSEDI, an RNA-targeted therapeutic, is the first treatment approved for Canadians living with hATTR amyloidosis, a disease caused by the abnormal formation of the transthyretin (TTR protein), resulting in TTR amyloid deposits in various tissues and organs throughout the body. The progressive accumulation of these deposits leads to sensory, motor and autonomic dysfunction, affecting multiple aspects of a patient's life.
“We appreciate Health Canada’s thorough and timely evaluation of TEGSEDI under priority review, and we look forward to working with all stakeholders to ensure that patients can receive timely and appropriate access to this important new treatment for Canadians living with hATTR amyloidosis,” said Jared Rhines, general manager at Akcea Therapeutics Canada. “On the heels of TEGSEDI’s marketing authorization in Europe, this news also represents an exciting milestone for Akcea with our first drug approval in Canada. We believe this achievement underscores Akcea’s commitment to the global rare disease community and our mission to deliver innovative therapies to patients no matter where they call home. We are excited to launch this innovative therapy along with Akcea ConnectTM, our comprehensive support program, for patients and their healthcare providers coast to coast.”
TEGSEDI is a once-weekly at-home subcutaneous injection that targets hATTR amyloidosis at its source by reducing the production of TTR protein.
“Today marks an exciting day for Canadians living with hATTR amyloidosis and their families as inotersen is the first approved disease-modifying therapy to address the significant burden many bear in living with debilitating and progressive symptoms. A tremendous emotional burden often comes with an inherited disease, and we believe this approval, and the innovation behind this treatment, means that today an important step in their hope for a brighter future has been realized. We now look forward to Akcea working with stakeholders to make this drug available to patients as soon as possible,” says Durhane Wong-Rieger, President and CEO, Canadian Organization for Rare Disorders (CORD).
"hATTR amyloidosis is a debilitating disease that carries with it significant morbidity and mortality, and we are extremely limited in options to offer patients with this disease and their families. The approval of inotersen changes this and is a welcome advance. In NEURO TTR, the study upon which Health Canada approval was sought, the changes from baseline showed statistically significant benefit in favor of inotersen treatment, including polyneuropathy symptoms and quality of life,” says Dr. Vera Bril, Professor of Medicine at the University of Toronto, Director of Neurology at University Health Network and Mount Sinai Hospital and the Krembil Family Chair in Neurology.
ABOUT TEGSEDITM (INOTERSEN INJECTION) (1)
TEGSEDITM (inotersen injection) is an antisense oligonucleotide (ASO) inhibitor of human transthyretin (TTR) production. TEGSEDI, discovered and developed by Ionis Pharmaceuticals, is also approved in the E.U. for the treatment of stage 1 or stage 2 polyneuropathy in adult patients with hereditary transthyretin amyloidosis (hATTR) and is currently under regulatory review in the U.S.
The approval is based on data from the NEURO-TTR study which was a Phase 3 randomized (2:1), double-blind, placebo-controlled, international study in 172 patients with hATTR amyloidosis with symptoms of polyneuropathy. The 15-month study measured the effects of TEGSEDI on neurological function and on quality-of-life by measuring the change from baseline in the modified Neuropathy Impairment Score +7 (mNIS+7) and in the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy (Norfolk QOL-DN) total score. TEGSEDI provided significant benefit on both of these co-primary endpoints in the NEURO-TTR study, including improvement in disease relative to baseline measurements in both co-primary endpoints for a substantial portion of patients as published in the New England Journal of Medicine (2).
Special Warnings and Precautions
TEGSEDI is associated with reductions in platelet count, which may result in thrombocytopenia. Platelet count should be monitored at least every two weeks during treatment with TEGSEDI and for 8 weeks following discontinuation of treatment.
Glomerulonephritis has occurred in patients treated with TEGSEDI. Careful monitoring of UPCR and eGFR is important during treatment with TEGSEDI.
The most common adverse reactions that occurred in at least 20% of TEGSEDI-treated patients and that occurred more frequently than on placebo were injection site erythema, nausea, fatigue, diarrhea, headache and injection site pain.
For more important safety information for TEGSEDI, including method of administration, drug interactions and adverse drug reactions, please see the Health Canada approved product monograph at www.akceatx.ca.
ABOUT HEREDITARY TRANSTHYRETIN (hATTR) AMYLOIDOSIS
hATTR amyloidosis is a progressive, systemic and fatal inherited disease caused by the abnormal formation of the TTR protein and aggregation of TTR amyloid deposits in various tissues and organs throughout the body, including in peripheral nerves, heart, intestinal tract, eyes, kidneys, central nervous system, thyroid and bone marrow. The progressive accumulation of TTR amyloid deposits in these tissues and organs leads to sensory, motor and autonomic dysfunction often having debilitating effects on multiple aspects of a patient's life. Patients with hATTR amyloidosis often present with a mixed phenotype and experience overlapping symptoms of polyneuropathy and cardiomyopathy.
Ultimately, hATTR amyloidosis results in death within three to fifteen years of symptom onset. Therapeutic options for the treatment of patients with hATTR amyloidosis are limited and, until this approval, there have been no disease-modifying drugs approved for the disease in Canada. There are an estimated 50,000 patients with hATTR amyloidosis worldwide.
About Akcea Therapeutics Canada
Akcea Therapeutics Canada, based in Ottawa Ontario, is the Canadian subsidiary of Akcea Therapeutics. Akcea is a biopharmaceutical company focused on developing and commercializing drugs to treat patients with serious and rare diseases. Akcea Therapeutics Canada is a member of Innovative Medicines Canada, the industry association representing Canada’s research-based pharmaceutical companies.
ABOUT AKCEA THERAPEUTICS
Akcea Therapeutics, Inc., an affiliate of Ionis Pharmaceuticals, Inc., is a biopharmaceutical company focused on developing and commercializing drugs to treat patients with serious and rare diseases. Akcea is advancing a mature pipeline of six novel drugs all with the potential to treat multiple diseases. All six drugs were discovered by and are being co-developed with Ionis, a leader in antisense therapeutics, and are based on Ionis’ proprietary antisense technology. TEGSEDITM (inotersen) is approved in Canada and the E.U. for the treatment of stage 1 or stage 2 polyneuropathy in adult patients with hereditary transthyretin amyloidosis (hATTR) and is currently under regulatory review in the U.S. WAYLIVRATM (volanesorsen) is under regulatory review for the treatment of familial chylomicronemia syndrome, or FCS, and is currently in Phase 3 clinical development for the treatment of people with familial partial lipodystrophy, or FPL. Akcea is a global company headquartered in Boston, Massachusetts with Canadian operations headquartered in Ottawa, Ontario. Additional information about Akcea is available at www.akceatx.com.
About Ionis Pharmaceuticals
As the leader in RNA-targeted drug discovery and development, Ionis has created an efficient, broadly applicable, proprietary antisense technology platform with the potential to treat diseases where no other therapeutic approaches have proven effective. Our drug discovery platform has served as a springboard for actionable promise and realized hope for patients with unmet needs – such as children and adults with spinal muscular atrophy (SMA). We created SPINRAZA® (nusinersen)* and are proud to have brought new hope to the SMA community by developing the first and only approved treatment for this disease.
Our sights are set on all the patients we have yet to reach with a pipeline of more than 40 drugs with the potential to treat patients with cardiovascular disease, rare diseases, neurological diseases, infectious diseases and cancer. We created TEGSEDI™ (inotersen) the world’s first RNA-targeted therapeutic approved for the treatment of stage 1 or stage 2 polyneuropathy in adult patients with hereditary transthyretin (TTR) amyloidosis (ATTR) that our affiliate Akcea Therapeutics is commercializing. Together with Akcea, we are also bringing new medicines to patients with cardiometabolic lipid disorders.
To learn more about Ionis follow us on twitter @ionispharma or visit http://ir.ionispharma.com/.
*Spinraza is marketed by Biogen.
AKCEA’S AND IONIS’ FORWARD-LOOKING STATEMENT
This press release includes forward-looking statements regarding the business of Akcea Therapeutics, Inc. and Ionis Pharmaceuticals, Inc. and the therapeutic and commercial potential of TEGSEDITM Any statement describing Akcea’s or Ionis’ goals, expectations, financial or other projections, intentions or beliefs, including the commercial potential of TEGSEDI or other of Akcea’s or Ionis’ drugs in development is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties, particularly those inherent in the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs. Akcea’s and Ionis’ forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its results to differ materially from those expressed or implied by such forward-looking statements. Although Akcea’s and Ionis’ forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known by Akcea and Ionis. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Ionis’ and Akcea’s programs are described in additional detail in Ionis’ and Akcea’s quarterly reports on Form 10-Q and annual reports on Form 10-K, which are on file with the SEC. Copies of these and other documents are available from each company.
In this press release, unless the context requires otherwise, “Ionis”, “Akcea,” “Company,” “Companies” “we,” “our,” and “us” refers to Ionis Pharmaceuticals and/or Akcea Therapeutics.
Ionis Pharmaceuticals™ is a trademark of Ionis Pharmaceuticals, Inc. Akcea Therapeutics™, TEGSEDITM and WAYLIVRATM are trademarks of Akcea Therapeutics, Inc.
Akcea Investor & Media Contact:
Kathleen Gallagher
Vice President of Communications and Investor Relations
phone 617-207-8509
e-mail: kgallagher@akceatx.com
-
Ionis Investor Contact:
D. Wade Walke, Ph.D.
Vice President, Investor Relations
phone 760-603-2741
e-mail: wwalke@ionisph.com
-
Ionis Media Contact:
Roslyn Patterson
Vice President, Corporate Communications
phone 760-603-2681
e-mail: rpatterson@ionisph.com
References
1. TEGSEDI™ Product Monograph
2. Benson et al., N Engl J Med 2018;379:22-31
An infographic accompanying this release is available at: http://resource.globenewswire.com/Resource/Download/dcb498ad-f1ce-4650-bc4e-0f64e8aed1a5
Copyright © 2018 GlobeNewswire, Inc.
Pobierz materiał i Publikuj za darmo
bezpośredni link do materiału
POBIERZ ZDJĘCIA I MATERIAŁY GRAFICZNE
Zdjęcia i materiały graficzne do bezpłatnego wykorzystania wyłącznie z treścią niniejszej informacji
| Data publikacji | 05.10.2018, 14:39 |
| Źródło informacji | GlobeNewswire |
| Zastrzeżenie | Za materiał opublikowany w serwisie PAP MediaRoom odpowiedzialność ponosi – z zastrzeżeniem postanowień art. 42 ust. 2 ustawy prawo prasowe – jego nadawca, wskazany każdorazowo jako „źródło informacji”. Informacje podpisane źródłem „PAP MediaRoom” są opracowywane przez dziennikarzy PAP we współpracy z firmami lub instytucjami – w ramach umów na obsługę medialną. Wszystkie materiały opublikowane w serwisie PAP MediaRoom mogą być bezpłatnie wykorzystywane przez media. |
Pozostałe z kategorii
-
Image

System, pacjent, technologia: XI Kongres Wyzwań Zdrowotnych uporządkuje kluczowe tematy przyszłości
Tegoroczny Kongres Wyzwań Zdrowotnych pod hasłem „Nowe strategie dla zdrowia. Czas na redefinicję celów i wyzwań” koncentruje się na konkretnych rozwiązaniach, które mają odpowiedzieć na wyzwania demograficzne, finansowe, technologiczne i klimatyczne. Udział w wydarzeniu potwierdziła Jolanta Sobierańska-Grenda, ministra zdrowia, a dyskusje będą poprowadzone w mocnym składzie reprezentantów różnych obszarów ochrony zdrowia. W agendzie znalazły się m.in. rozmowy o reformie szpitali, granicach wytrzymałości systemu czy bezpieczeństwie w kontekście zmian geopolitycznych. Eksperci poruszą także tematy wynagrodzeń kadr i profilaktyki. Nie zabraknie również perspektywy pacjentów. Rejestracja na wydarzenie jest otwarta.- 18.02.2026, 15:27
- Kategoria: Zdrowie i styl życia
- Źródło: Polskie Towarzystwo Wspierania Przedsiębiorczości
-
Image

Głos nauki, przekaz mediów: partnerstwo na rzecz walki z zaburzeniami lipidowymi
Okrągły stół z dziennikarzami o cholesterolu, nowych wytycznych, współpracy międzynarodowej i roli interdyscyplinarnej opieki.- 17.02.2026, 15:38
- Kategoria: Zdrowie i styl życia
- Źródło: Polskie Towarzystwo Lipidologiczne
Newsletter
Newsletter portalu PAP MediaRoom to przesyłane do odbiorców raz dziennie zestawienie informacji prasowych, komunikatów instytucji oraz artykułów dziennikarskich, które zostały opublikowane na portalu danego dnia.
ZAPISZ SIĘ